Information Details
What is FDA registration? What are the requirements for FDA registration?
Release time:
2024-03-20 15:48
FDA (U.S. Food and Drug Administration) is an important agency of the U.S. government responsible for regulating the safety and effectiveness of food, drugs and medical devices. If you want to sell food, drugs or medical devices in the United States, you must be registered and approved by the FDA. This new Angrun consultation will detail the FDA registration process and related requirements.
FDA registration is a rigorous and cumbersome process. Applicants first need to determine the classification of the product before starting registration. Depending on the type of product, the FDA classifies it into three categories: food, drug, or medical device. The registration process is different for each category and is described separately below.
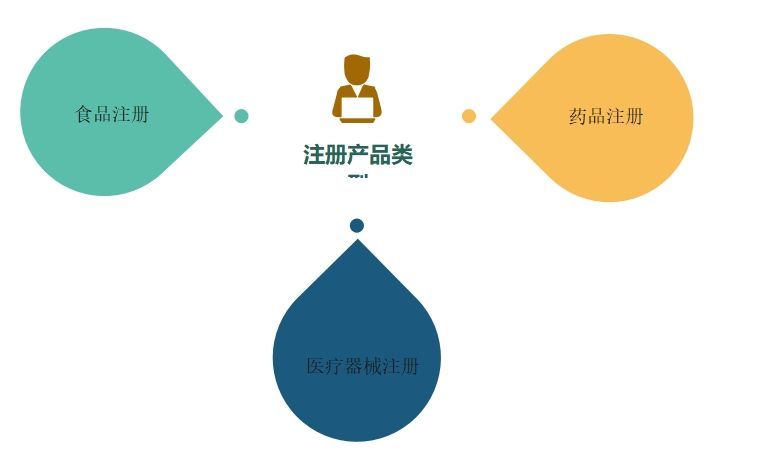
1. Food Registration
Food registration applies to food products sold in the U.S. market, including packaged foods, beverages, and dietary supplements. When registering food, detailed product information is required, including ingredients, production quality control and safety assessment. At the same time, also need to provide packaging labels and advertising materials and other related documents.
2. Drug Registration
Drug registration applies to drugs sold in the U.S. market, including prescription and over-the-counter drugs. The drug registration process is more complex, requiring detailed clinical study data, drug composition and manufacturing process information. In addition, approval through the FDA's New Drug Application (NDA) or General Drug Application (ANDA) is required.
3. Medical Device Registration
Medical device registration applies to medical devices sold in the U.S. market, such as monitoring instruments, surgical instruments, and cardiac pacemakers. The registration process requires detailed technical specifications, performance data, and clinical trial results. Registration requirements vary depending on the risk level of the device.
The above is the basic process of FDA registration, but to successfully obtain FDA registration and approval, the following requirements must be met:
1. Production quality control
FDA attaches great importance to production quality control. Applicants need to provide a sound production process and quality system to ensure product reliability and safety. In addition, there is a need to develop plans for emergency recalls and adverse event reporting, as well as to establish a sound quality tracking system.
2. Clinical research and laboratory testing
For drugs and medical devices, clinical studies and laboratory tests are needed to prove their effectiveness and safety. Applicants are required to prepare a detailed study protocol and obtain approval from an ethics committee and regulatory body.
3. Registration Fees
FDA registration is subject to a registration fee, which is related to the type of product and the complexity of the registration. Applicants need to know and prepare the registration fee in advance to ensure a smooth registration process.
In short, FDA registration is the only way to enter the US market. Applicants need to understand and meet the relevant registration process and requirements. During the registration process, communication and cooperation with FDA are also very important, and the required information and materials are provided in a timely manner to speed up the registration process. Only through FDA registration and approval can food, drugs and medical devices be legally sold in the US market to ensure the safety and effectiveness of products.
Previous Page
Previous Page








