Cosmetic raw material information submission
Cosmetic raw material information submission
1. regulatory background
Article 27 of the Code for Registration and Filing Materials of Cosmetics (draft for comments, 2020/11/4) (II) stipulates that "(raw material safety related information) the registrant, the filing person or the domestic responsible person shall fill in the manufacturer information of the raw materials used in the products and upload the raw material quality and safety information documents issued by the raw material manufacturer. If the raw material manufacturer has submitted information on the quality and safety of raw materials in accordance with the Guidelines for Submitting Information on the Quality and Safety of Cosmetic Raw Materials (Annex 12 and Annex 13), the registrant, the filing person or the domestic responsible person may fill in the information document on the quality and safety of raw materials submitted by the raw material submission code (Annex 14)".
The submission code of cosmetic raw materials requires that each raw material produced by each production enterprise only corresponds to one submission code, which is equivalent to the ID card of the raw material. If a cosmetic product company chooses an enterprise that has not obtained the submission code, it may not be able to obtain the safety information file of its raw materials, which will directly affect the registration and filing of cosmetic products.
2. submission process
1. Make judgments on existing raw materials and new raw materials for cosmetics
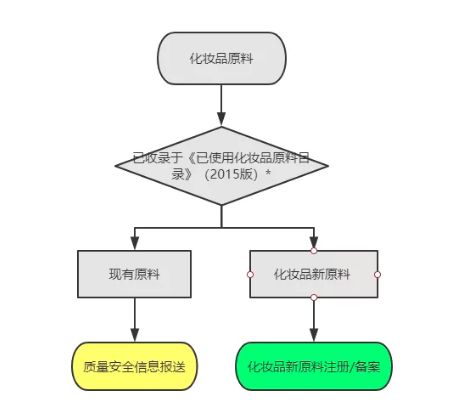
Figure 1 Judging the existing raw materials and new cosmetic raw materials according to the Catalogue of Used Cosmetic Raw Materials (2015 Edition)
2. Quality and safety information management of raw materials in cosmetics registration
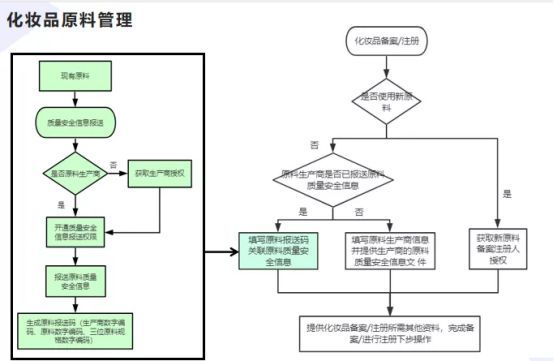
Figure 2 Quality and safety information management of raw materials in cosmetics registration
3.Guidelines for Submission of Official Cosmetic Raw Material Safety Information
The manufacturer of (I) cosmetic raw materials refers to the enterprise that is responsible for the safety of raw materials, which can be the actual production enterprise of raw materials, the affiliated enterprise belonging to the same group company as the actual production enterprise of raw materials, or the entrusted enterprise in the entrusted production of raw materials.
Cosmetic raw material manufacturers shall submit the "Raw Material Safety Information Filing Enterprise Information Form" and the main body certification documents of the enterprise through the raw material safety information service platform of the State Drug Administration, and open the authority to submit information related to the safety of cosmetic raw materials.
(II) overseas or domestic raw material manufacturers may submit the safety information of cosmetic raw materials by themselves, and may also authorize overseas or domestic legal person enterprises to submit and maintain the safety information of raw materials. When the authorized enterprise opens the user right, it shall also submit the power of attorney issued by the manufacturer of cosmetic raw materials at the same time. The authorization letter shall specify the authorization relationship and scope of authorization, and only one enterprise can be authorized with the raw material safety-related information of the same quality specification.
The safety-related information of (III) raw materials shall include the trade name of the raw material, basic information of the raw material, a brief description of the raw material production process, necessary quality control requirements, assessment conclusions of international authoritative institutions, and limited requirements for risk substances.
Cosmetic production water without the need to submit raw material safety-related information, except for water from special origin.
After the safety-related information of (IV) raw materials is submitted through the information platform, the raw material submission code is automatically generated. The raw material submission code consists of five manufacturer digital codes, six raw material digital codes and three raw material quality specification digital codes, and each group of codes is connected.
The State Drug Administration of (V) shall take the initiative to disclose the trade name, manufacturer information and raw material submission code of the raw material that has submitted the safety information of the raw material.
Cosmetics registrants, filers or domestic responsible persons may fill in the raw material submission code related raw material safety information documents when applying for special cosmetics registration or handling ordinary cosmetics filing. When the raw material manufacturer or safety-related information changes, the registrant, the filing person or the domestic responsible person shall change the relevant information in a timely manner.
4.Raw material information submission transition policy
NMPA has developed relevant transitional measures and determined the data requirements for raw material submission codes. Cosmetics enterprises should make corresponding preparations in advance according to the transitional period policy.
Starting from May 1, 2021, when applying for registration or filing, the registrant/filer shall fill in the source and trade name information of the raw materials of the product formula, which involves the raw materials with quality specifications required in the Cosmetic Safety Technical Specification, and shall also submit the quality specification certificate or safety-related information of the raw materials.
Starting from January 1, 2022, when applying for registration or filing, the registrant/filer shall provide safety-related information on raw materials with anti-corrosion, sun protection, coloring, hair dyeing, freckle and whitening functions in accordance with the requirements of the Regulations.
From January 1, 2023, when applying for registration or filing, the registrant/filer shall provide safety-related information on all raw materials in accordance with the requirements of the Regulations. For cosmetics that have been registered or filed before, the registrant and the filing person shall provide additional safety-related information on all raw materials in the product formula before May 1, 2023.
5.Countermeasures of raw material enterprises and cosmetics enterprises
(1) Cosmetic raw material enterprises
As soon as possible to complete the quality and safety information of raw materials, for the downstream cosmetics enterprise product registration to provide convenience. Under the new system, the quality and safety information/submission code of raw materials shall be provided simultaneously during the filing/registration of cosmetics.
(2) Cosmetic finished products enterprises
Organize the existing raw material inventory. If there are the same raw materials produced by different raw material manufacturers, organize the internal raw material system or raw material code as needed to meet the requirements of subsequent new regulations. Under the new regulations, cosmetics should be directly associated with raw material manufacturers when filing and registering. If raw material manufacturers need to be changed after filing, raw material information should be maintained through the filing and registration platform, and safety assessment should be carried out if necessary.
6. information Q & A
Q1:If the quality and safety information of cosmetic raw materials is not submitted within the stipulated time, what are the penalties?
Answer: The submission of quality and safety information of cosmetic raw materials can be provided by the raw material manufacturer or the cosmetic enterprise that uses this raw material during the product registration and filing. If the manufacturer does not submit the information in the early stage, there is no submission code. If the finished product registration and filing cannot be related to relevant information through the submission code, then the finished product registration and filing must be filled in.
If it is not filled in, the registration of the whole finished product cannot be carried out.
Q2:What are the specific requirements for the notarization of a power of attorney? The system is not yet open, and what are the current regulations?
A: According to the information communicated at the training meeting of the High Research Institute of the Food and Drug Administration on April 22-23, the authorization letter submitted for the quality and safety information of cosmetic raw materials does not need to be notarized.
The system is not open. At present, the quality and safety information of raw materials can only be filled in when the finished products of cosmetics are registered.
Q3:If the ingredient list of cosmetic raw materials contains residues that do not belong to functional ingredients, such as 1% glucose and 2% solvent residues, if all the ingredients are marked with impurities, the label will be very long, can it not be marked on the cosmetic label?
Answer: According to the standard requirements for labeling the ingredient list on cosmetic labels, these need to be labeled. However, it can be compared with the "full ingredient list-labeling exemption".
Full Ingredient Table-Note Exemptions are divided into the following five points:
1, to protect the raw materials and add the right amount of preservatives, antioxidants and other ingredients. Because the amount of raw materials added is limited or relatively small, the content of preservatives, antioxidants and other ingredients brought in by raw materials in the final cosmetics is far less than the limit requirements of cosmetic hygiene standards, so labeling can be exempted;
2. Trace impurities mixed in raw materials. Under the current technical conditions, these trace impurities inevitably exist in the raw materials. The existence of these trace impurities does not affect the safety evaluation and use of the raw materials:
3. Trace unreacted substances or reaction by-products present in the reaction product:
4. Processing aids intentionally added in the production process but not present in the final product:
5, as a mask and other products of the carrier of non-woven fabric is not considered cosmetic ingredients.
Q4:Regarding prohibited plants, if I do not confirm whether a plant can be used, is there any organization that can inquire?
Answer: The substances in the list of prohibited plants must not be used. The plant raw materials in the list of used cosmetic raw materials can be used. If you are not sure whether they belong to the list, you can find a consulting organization or industry experts to judge, but there should be no special organization to issue a conclusion on the report format at present. If it is finally confirmed that it is not included in the used cosmetic raw materials, new raw materials registration is required.
Q5:Cosmetic raw materials do not have a production license, and distributors may fill in their own products according to the manufacturers. If the raw material information reporting system is opened, how can manufacturers and distributors be distinguished?
Answer: The submission of cosmetic raw materials should be carried out by the manufacturer or the person authorized by the manufacturer. The submission code can only be obtained after the raw materials are submitted.
Q6:The delivery of raw materials does not need notarization, and the registration and filing of new raw materials needs notarization. Is this right?
Answer: The power of attorney submitted for raw material quality and safety information does not need to be notarized.
For the registration and filing of new raw materials, if the registered filing person is abroad, it is necessary to authorize the domestic responsible person to carry out the registration and filing, and the authorization letter needs to be notarized.
Q7:Who carries out the quality and safety information of cosmetic raw materials?
Answer:According to the Regulations on the Administration of Cosmetic Registration and Filing Materials, cosmetic raw material manufacturers can submit their own cosmetic raw material safety information, and can also authorize overseas or domestic legal person enterprises to submit and maintain raw material safety information. Only one enterprise can be authorized for raw material safety information of the same quality specification.
Cosmetic raw material manufacturer refers to the enterprise that is responsible for the safety of raw materials, which can be the actual production enterprise of raw materials, the affiliated enterprise belonging to the same group company as the actual production enterprise of raw materials, or the entrusted enterprise in the entrusted production of raw materials. The raw material distributor shall judge whether it meets the definition of raw material manufacturer according to the actual situation.
The reporting platform is divided into domestic users and overseas users. Domestic users log in directly through the "Cosmetic Raw Material Safety Information Registration Platform" module in the online service hall of the State Food and Drug Administration. Overseas users log in after opening the account number of the "Cosmetic Raw Material Safety Information Registration Platform.
Among them, if the overseas raw material manufacturer submits it by itself or authorizes the overseas enterprise to submit it, the main body certification document of the raw material manufacturer shall be notarized by the Chinese notary office or certified by the Chinese embassy (consulate), and the relevant documents shall be uploaded together with the main body certification document. If the overseas raw material manufacturer authorizes the domestic enterprise to submit the report, the domestic authorized enterprise shall verify the main body and relevant information of the overseas raw material manufacturer, and after the authorized enterprise opens the account, it shall submit the main body certification document of the cosmetic raw material manufacturer and the power of attorney issued by the raw material manufacturer at the same time. The authorization letter shall specify the authorization relationship and scope of authorization, and only one enterprise can be authorized with the raw material safety-related information of the same quality specification.
Responsibility of the authorized enterprise: when the authorized enterprise opens the user right, it shall submit the authorization letter issued by the manufacturer of cosmetic raw materials at the same time. The responsibility of the authorized enterprise is relatively limited. It can only submit and maintain the safety information of raw materials. It can be a third-party consulting organization, which will not have a great impact on the actual trade of raw materials of the authorized enterprise.
Q8:What does the raw material attribute information refer to? How to choose?
Answer: The attributes of raw materials include the basic characteristics of the main components, the source of raw materials, the production process and other information, which are mainly judged based on the production process of raw materials, starting materials, etc. From this, it can be seen that the process is the key requirement in the submission of raw material quality and safety information.
Q9:What do the characteristic indicators need to be filled in?
Answer: In the characteristic index of raw materials, the single compound with clear structure, polymer raw material, plant raw material (direct source), peptide, protein, nano raw material, other and other options are listed respectively. The submitter should describe its characteristic index according to the specific product category.








