Raw and Auxiliary Package Registration
Registration of Imported and Domestic APIs, Pharmaceutical Excipients and Pharmaceutical Package Materials in 1.
Legal basis |
"the People's Republic of China Drug Administration Law" "the People's Republic of China Drug Administration Law Implementation Regulations" "Drug Registration Management Measures" Announcement of the General Administration of the People's Republic of China on Adjusting the Examination and Approval of Raw Materials, Pharmaceutical Excipients and Pharmaceutical Package Materials (No. 146 of 2017) Announcement of the State Food and Drug Administration on Matters concerning Further Improving the Review, Approval and Supervision of Drug Related Issues (No. 56 of 2019) |
| Scope of application | Excipients developed, produced, imported and used in the People's Republic of China. |
| Registration Data Requirements | It shall comply with the requirements of Annex 1 in the announcement of the General Administration on adjusting the review and approval of bulk drugs, pharmaceutical excipients and pharmaceutical packaging materials. |
| Registration Type | Import accessories registration, update, annual report. Domestic accessories registration, update, annual report. |
| Acceptance institutions | Center for Drug Evaluation (CDE), State Drug Administration |
Registration target of imported and domestic accessories in 2.
Provide reliable registration consulting services for customers' registration of imported or domestic excipients, assist customers' products to successfully pass the technical review when reviewing related to preparation products, so that preparation enterprises can obtain the "Import Drug Registration Certificate"/"Pharmaceutical Product Registration Certificate" or drug approval number, thus realizing CDE to mark the excipient registration number "through technical review", I .e. "A" status.
3. service content
1. Provide registered agent services for imported accessories.
|
4. Review, translation, perfection, compilation and submission of registration materials | 7. Technology transfer of auxiliary materials registration data. |
2. Provide an outline for the preparation of the declaration information.
|
5. Registration inspection application, sample delivery, tracking and problem solving | 8. Submission of the annual report of registered excipients. |
3. Gap analysis of the registration data, estimate the risk, and propose corrective measures to make the registration accessories pass the technical review smoothly.
|
6. Follow up the whole process of auxiliary materials registration, send supplementary questions and answer and submit supplementary reply materials. | 9. Update of auxiliary materials registration data, including major changes, medium changes, minor changes and basic information changes. |
4. Imported and Domestic Accessories Registration Process

5. China's Drug Package Material Regulatory Standards and Models
As an important part of the national drug standard system, the Chinese Pharmacopoeia, together with the promulgation of the the People's Republic of China Drug Administration Law, the Implementation Regulations of the the People's Republic of China Drug Administration Law, the Requirements for Registration of Drug Packaging Materials and various technical guidelines, together with the national drug packaging material standards (referred to as YBB standards), constitute the regulatory system for the supervision of drug packaging materials. According to the contents of the announcement of the State Drug Administration (Announcement No. 56 of 2019), China's supervision of drug packaging materials has changed from the previous registration management system to a registration system, and related technical reviews are conducted with pharmaceutical preparations or APIs, making the approval review more efficient and scientific.
Registration process of drug packaging materials
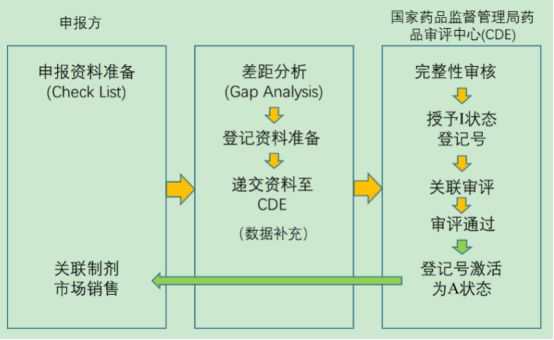
Registration steps of 6. packaging materials
· Fill in the basic information of drug packaging materials on the CDE portal and submit the registration data to CDE in the form of CD-ROM;
· CDE conducts a completeness review of the registration data within 5 working days of receipt of the data;
· When the data are incomplete, the drug packaging enterprises are required to supplement the registration data until they meet the requirements. CDE will publicize the relevant registration information and grant I status registration number (registration number consists of B four-digit year number and seven-digit serial number);
· When the package material passes the associated review, the registration number is activated to A status.
7.Requirements for registration data of pharmaceutical excipients
The Announcement of the State Food and Drug Administration on Matters Related to Further Improving the Review, Approval and Supervision of Drugs (No. 56 of 2019) divides drug packaging materials into the following categories according to their use:
There is no history of use in domestic and foreign listed drugs, including
○ Accessories 1.1 new molecular structures and accessories that do not belong to 1.2 and 1.3;
○ The 1.2 is changed by simple chemical structure (such as salt base, hydrate, etc.) by excipients with a history of use;
○ Excipients obtained by co-processing of excipients with a history of use of both and more than two 1.3;
○ Excipients that have been used in 1.4 but have changed the route of administration.
There is a history of use in domestic and foreign listed drugs, and.
○ 2.1 excipients not included in Chinese Pharmacopoeia/USP/EP/BP/JP;
○ Excipients that have been included in one of the 2.2 USP/EP/BP/JP but are not used in domestic listed drugs;
○ Excipients that have been included in one of the 2.3 USP/EP/BP/JP but not in the Chinese Pharmacopoeia;
○ 2.4 the excipients included in the Chinese Pharmacopoeia.
Has a history of use in food or cosmetics, and
○ 3.1 excipients for oral preparations with national food safety standards;
o 3.2 excipients for external use preparations having national or industrial standards for cosmetics.
○ Other
The proposed route of administration of the preparation: injection, inhalation, eye use, local and sublingual transdermal administration, oral administration, other
Source: ○ animal or human ○ mineral ○ plant ○ chemical synthesis ○ microbial fermentation or bioengineering ○ others
Registration Information Form of Pharmaceutical Excipients
Information Item |
Content |
1.1* |
1.2* |
1.3* |
1.4* |
2.1* |
2.2* |
2.3* |
2.4* |
3.1* |
3.2* |
1 |
Basic information of registrant |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
2 |
Basic information of accessories |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
3 |
3.1(13) Process Overview |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
3.1(22) Process Detail |
+ |
± |
± |
± |
± |
± |
- |
- |
± |
± |
|
3.1(3) Explain the batch principle, batch range and basis for commercial production. |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
3.1(41) Equipment |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
3.2.1Key material control information |
- |
- |
- |
+ |
- |
- |
+ |
+ |
+ |
+ |
|
3.2.2Material Control Information Detail |
+ |
+ |
+ |
- |
+ |
+ |
- |
- |
- |
- |
|
3.3Control of key steps and intermediates |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ |
|
3.4.1Process Stability Assessment |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
3.4.2Process Validation |
+ |
+ |
+ |
- |
- |
- |
- |
- |
- |
- |
|
3.5Development of production processes |
+ |
± |
± |
- |
± |
± |
- |
- |
- |
- |
|
4 |
4.1.1(11) Structural Confirmation Information |
+ |
+ |
+ |
+ |
+ |
+ |
± |
- |
+ |
+ |
4.1.1(21) Structural Confirmation Study |
+ |
± |
+ |
- |
- |
- |
- |
- |
- |
- |
|
4.1.2physical and chemical properties |
+ |
± |
± |
± |
± |
± |
- |
- |
± |
± |
|
4.2.1Impurity information |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
4.2.2impurity study |
+ |
± |
± |
± |
± |
± |
- |
- |
± |
± |
|
4.3.1Functional characteristic information |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
4.3.2functional characteristics study |
+ |
+ |
+ |
± |
± |
± |
- |
- |
± |
± |
|
5 |
5.1Quality Standards |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
5.2Validation of analytical methods |
+ |
+ |
+ |
± |
+ |
+ |
- |
- |
± |
± |
|
5.3Quality standard formulation basis |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
6 |
Batch Inspection Report |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
7 |
7.1Stability Summary |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
7.2Stability data |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
7.3Packaging of accessories |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
8 |
Pharmacological and Toxicological Research |
+ |
+ |
+ |
+ |
+ |
± |
± |
± |
± |
± |
Note:+Items for which relevant information is required
-Items for which no relevant information is required
±Items for which relevant information is provided as required
Remarks: *
There is no history of use in domestic and foreign listed drugs, including
Accessories 1.1 new molecular structures and accessories that do not belong to 1.2 and 1.3;
The 1.2 is changed by simple chemical structure (such as salt base, hydrate, etc.) by excipients with a history of use;
1.3 excipients obtained by co-processing of two or more excipients with a history of use;
1.4 excipients that have a history of use but have changed the route of administration.
There is a history of use in domestic and foreign listed drugs, and.
2.1 excipients not included in Chinese Pharmacopoeia/USP/EP/BP/JP;
Excipients that have been included in one of the 2.2 USP/EP/BP/JP but are not used in domestic marketed drugs;
Excipients that have been included in one of the 2.3 USP/EP/BP/JP, but not in the Chinese Pharmacopoeia;
2.4 the excipients that have been included in the Chinese Pharmacopoeia.
Has a history of use in food or cosmetics, and
3.1 excipients for oral preparations with national food safety standards;
Excipients for external use preparations having national or industrial standards for cosmetics are 3.2.
Note:
(1) High-risk pharmaceutical excipients generally include: pharmaceutical excipients of animal or human origin; pharmaceutical excipients for injections, eye preparations, inhalation preparations, etc. The registration data requirements for high-risk excipients can be provided on demand according to the application of excipients in specific preparations and the corresponding technical requirements, or supplemented during the review process according to the application of specific preparations and excipients in preparations.
(2) For excipients with a history of use, if the excipients exceed the historical maximum usage of the corresponding route of administration, relevant safety data and other information shall be provided.
(3) For premixed excipients, the appropriate information requirements should be selected for registration according to their application in the preparation and the composition of each excipient in the formulation.
(4) The above registration data classification requirements serve as guidance for the preparation of information for registrants, and the Drug Evaluation Center may make additional information requirements based on the technical review needs of the preparation.
(5) According to the classification of auxiliary materials, a group of research data can be provided in the registration data 3.2.1 and 3.2.2,3.4.1 and 3.4.2,4.1.1(1) and (2).
8. our services.
· Registration and consultation of drug packaging materials
· Drug packaging materials registration business agent
· Translation of registration materials
· Follow-up of Drug Package Material Association Review
· Annual report of pharmaceutical packaging materials
· Laboratory entrustment and test supervision
· Official inquiry communication








