Cosmetics new raw materials for the record registration
Cosmetics New Raw Material Filing/Registration
1. scope of application
New raw materials for cosmetics produced and operated in the People's Republic of China.
What is a new cosmetic material?
According to the Regulations on the Supervision and Administration of Cosmetics (State Order No. 727), the natural or artificial raw materials used in cosmetics for the first time in my country are new raw materials for cosmetics. The registered and filed new raw materials for cosmetics are still used before they are included in the catalog of used cosmetic raw materials. New raw materials for cosmetics are managed.
It should be noted that only when the expected use method, use part and use purpose of the raw materials meet the relevant attributes of cosmetics, can they apply for registration or record according to the new raw materials of cosmetics.
If the use method of a certain raw material is taken orally or injected, which does not conform to the description of the use method of cosmetics in the "Regulations", that is, "rubbing, spraying or other similar methods", or the use part and purpose of the raw material do not belong to cosmetics If the definition category, you cannot apply for registration or filing according to the new cosmetic raw materials.
At the same time, according to the requirements of the regulations on the Administration of Registration and filing materials of New Cosmetic raw materials, the registration and filing materials of new cosmetic raw materials should be based on scientific research and objectively and accurately describe the characteristics, characteristics and safe use requirements of new raw materials.
The ingredients of new cosmetic raw materials applied for registration or filing shall be relatively clear, and the registrant, filing person or domestic responsible person shall submit the registration and filing materials of new cosmetic raw materials as required, and shall be responsible for the legality, authenticity, accuracy, completeness and traceability of the submitted materials.
In line with what circumstances do not belong to the new raw materials of cosmetics?
Raw materials that meet one of the following conditions are not new raw materials for cosmetics:
1. Ingredients included in the Catalogue of Used Cosmetic Ingredients (2021 Edition).
When selecting the raw materials in the catalog, the registrant and the filing person of cosmetics shall comply with the relevant requirements of relevant national laws and regulations, mandatory national standards, and technical specifications, and bear the responsibility for product quality and safety. If it is required to exceed the "maximum historical usage", its safety should be proved in accordance with the procedures and requirements of the "Technical Guidelines for Cosmetic Safety Evaluation.
2. The specific raw materials contained in the used category of raw materials.
Such as the catalog has been included in the category of raw materials "collagen", that is, collagen, expressed as a general term for a category of raw materials, the category of raw materials contains different process sources such as animal tissue extraction, genetic recombination of collagen, but also contains different types such as type I collagen, type III collagen and so on. In addition, the "so-and-so plant extract" raw materials included in the catalogue of used cosmetic raw materials (2021 edition), such as "ginseng extract", indicate that the whole ginseng plant and its extract are used raw materials. if the "ginseng juice" or a specific part of ginseng is declared as a new raw material, it will not be accepted.
3. "Cosmetic Safety Technical Specification" has been specified as the raw material of the banned component.
Such as human cells, tissues or human products; antihistamines; hormone substances.
4. Raw materials whose actual function is beyond the definition of cosmetics.
Such as "activated cells", "regenerative cells", "reduce the pigmentation of the wound site", "promote healing", "promote the efflux of heavy metals" and other raw materials with medical effects.
2. policies and regulations
1. Regulations on the Supervision and Administration of Cosmetics (Order No. 727 of the State Council of the People's Republic of China)
Content links:https://www.nmpa.gov.cn/xxgk/fgwj/flxzhfg/20200629190501801.html
2、Measures for the Administration of Cosmetics Registration
Content links:https://www.nmpa.gov.cn/xxgk/fgwj/flxzhfg/20210112114521164.html
3. Provisions on the Administration of Registration and Filing Data of New Raw Materials for Cosmetics
Content links:https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20210304140454159.html
4. Provisions on the Administration of Registration and Filing Materials of Cosmetics
Content links:https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20210304140747119.html
3. filing/registration process
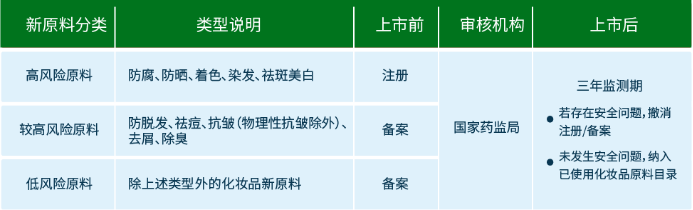
According to the "Regulations", the state implements classified management of cosmetic raw materials according to the degree of risk, implements registration management of new cosmetic raw materials with a higher degree of risk, and implements record management of new cosmetic raw materials. The filing of new raw materials for cosmetics shall be completed after the filing materials stipulated in these regulations are submitted by the online government service platform of the drug regulatory department of the State Council.
The real meaning of the record is that the new raw material filer submits the information to the drug regulatory department for reference. After the filing of the new raw material for cosmetics is completed, the State Food and Drug Administration publishes the filing information of the new raw material, which only means that the raw material has completed the submission of the filing information and meets the formal requirements, while the authenticity, scientificity and adequacy of the information content may not be verified. The disclosure of information about the new cosmetic raw materials that have been filed does not mean that the safety and functionality of the new raw materials are recognized, let alone the statement of "successful approval for filing.
According to the regulations and measures, after the filing of new cosmetic raw materials is completed, the drug supervision and administration department will organize technical review institutions to carry out technical verification on the filing materials of new raw materials, and track and evaluate the use and safety of new cosmetic raw materials. If it is found that the filing materials of new cosmetic raw materials do not meet the requirements, it will be ordered to make corrections within a time limit.
Among them, if the filing materials related to the safety of new cosmetic raw materials do not meet the requirements, the sales and use of new raw materials may be ordered to be suspended at the same time; if it is found that new cosmetic raw materials do not fall within the scope of filing, or false materials are submitted during filing, the filing of new cosmetic raw materials will be canceled; new cosmetic raw materials are ordered to suspend use or cancel filing, cosmetics registrants and filers shall suspend or stop the production and operation of cosmetics using the new raw materials at the same time.
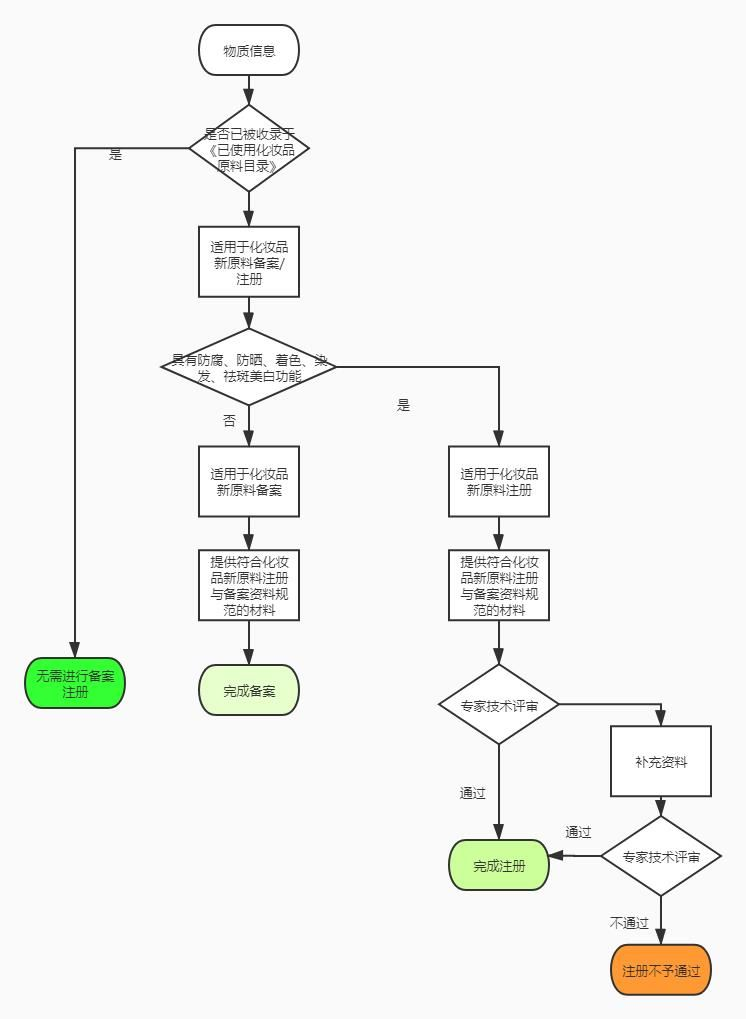
How to determine that new cosmetic raw materials should apply for registration or filing according to the attributes of new cosmetic raw materials?
According to the regulations, new cosmetic raw materials with anti-corrosion, sun protection, coloring, hair dyeing, freckle removing and whitening functions can only be used after being registered by the drug regulatory department of the State Council; other new cosmetic raw materials shall be filed with the drug regulatory department of the State Council before use. This is based on the principle of risk management, the relatively high risk of several types of raw materials to implement registration management, other raw materials to implement record management.
In the process of developing new cosmetic raw materials, it is often found that a new raw material may have multiple functions at the same time. Before applying for registration or filing, the registrant and the filing person of the new cosmetic raw material shall conduct a comprehensive review and full study of the actual functions that the new raw material may have, and make a scientific and reasonable judgment on whether the new raw material belongs to the situation that should be declared for registration.
Generally speaking, for new raw materials with multiple functions at the same time, as long as one of the functions belongs to the situation that should be declared and registered, the new raw materials should be declared and registered in accordance with the requirements of the regulations, and can be used only after approval and registration; if the multiple functions at the same time do not belong to the situation that should be declared and registered, regardless of the types of functions, it can be filed with the State Food and Drug Administration before use. The registrant and the filing person of the new raw materials for cosmetics shall not intentionally conceal the actual functions of the new raw materials, and shall not use the new raw materials for cosmetics that should be declared for registration only for the record and then for the production of cosmetics. Once such acts are verified, they will be punished in accordance with the provisions of paragraph 3 of Article 59 of the regulations.
4. Information Requirements
In order to cooperate with the implementation of the "Regulations on the Supervision and Administration of Cosmetics" and the "Administrative Measures for the Registration and Filing of Cosmetics", and to standardize and guide the registration/filing of new cosmetic raw materials, the State Drug Administration issued the "Specifications for the Registration and Filing of New Cosmetic Raw Materials". According to the requirements of this regulation, the application for registration or filing needs to submit the information of the registered filing person, the overview of the security risk monitoring and evaluation system, the information of the domestic responsible person and the authorization documents (the registrant filing person is overseas) for user registration, and then submit the relevant information.
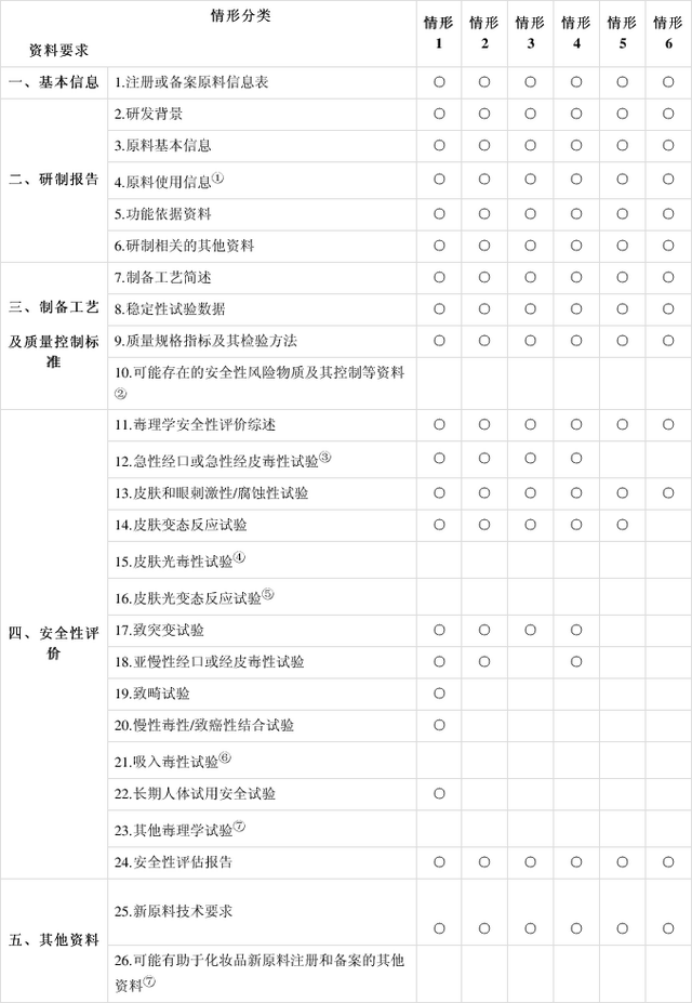
Note: "○" in the above table indicates that the information must be submitted. The meaning of the upper corner markings required for data items is explained as follows:
① For new raw materials with a history of use abroad, the use of raw materials in cosmetics abroad should be explained.
② The new raw materials of possible safety risk substances shall be submitted.
③ In case 3, if the safety assessment report or the human safety inspection report under the ethical conditions can be provided at the same time that the international authoritative safety evaluation organization concludes that it is safe to use in cosmetics, the information may not be submitted.
④ The test data shall be submitted when the raw material has ultraviolet absorption characteristics.
⑤ Except for case 6, the test data shall be submitted when the raw material has ultraviolet absorption characteristics.
⑥ The information shall be submitted when the raw material is likely to be exposed by inhalation.
⑦ Other materials submitted according to the actual situation of each new raw material.
After the registration of new raw materials is completed, what obligations should be fulfilled by the registrant and the filing person of new raw materials for cosmetics?
Answer: According to the "Regulations" and "Measures", the registrant and filer of new cosmetics raw materials are responsible for the quality and safety of new cosmetics raw materials. The new cosmetic raw materials that have been registered and completed the record shall be subject to the safety monitoring period system. During the safety monitoring period, the registrant and the archivist of the new cosmetic raw materials shall pay close attention to the safe use of the new raw materials, collect and sort out the relevant information on the use of the new raw materials in accordance with the requirements of the Regulations, and prepare the Annual Report on Safety Monitoring of New Cosmetic Raw Materials. Within 30 working days before the safety monitoring of new cosmetic raw materials, submitted to the technical review body through the information service platform.
If the registrant or recordholder of new cosmetic raw materials discovers that there is a situation that should be reported to the technical review agency in the use of new raw materials in the "Measures", or other situations that are deemed necessary to be reported, they shall immediately prepare the "New Cosmetic Raw Material" in accordance with the requirements of the "Regulations". "Safety Risk Control Report" shall be submitted to the technical review agency through the information service platform.
5. service content
1. Cosmetic raw material quality and safety information submission service
2. Supervision services for physicochemical/toxicological/efficacy tests of new cosmetic materials
3. Cosmetic new raw material safety assessment service
4. Tracking the whole process of filing/registration of new cosmetic raw materials








