Food contact materials and food registration
1. what are food contact materials and products
 (1) Definition of food contact materials and products
(1) Definition of food contact materials and products
Under normal conditions of use, materials and articles that have been or are expected to be in contact with food or food additives, or whose components may be transferred to food.
Includes:Packaging materials, containers, tools and equipment used for food in the production, processing, packaging, transportation, storage, sale and use of food; inks, adhesives, lubricants, etc. that may directly or indirectly contact food.
Not Included: Detergents; Disinfectants; Public Water Delivery Facilities.
(2) Food contact materials and products vs food-related products
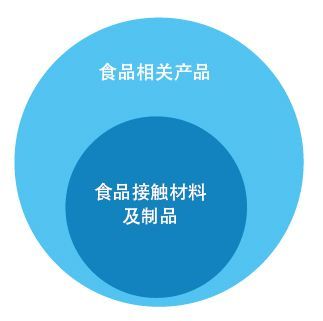
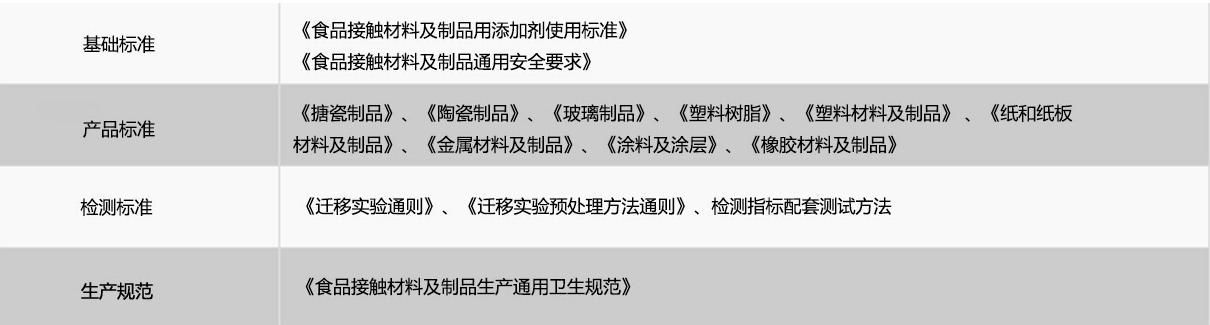
Regulations on Food Contact Materials and Products in China, 2.
3. Food Contact Materials and Articles Compliance Obligations
1. Which substances need to issue a declaration of conformity?
(1) Substances permitted in national standards
(2) Substances approved for use in the notice of the Health and Wellness Commission
Which substances need to be declared as new substances?
(1) Food packaging materials, containers and additives that have not been included in the national food safety standards or permitted by the Ministry of Health.
(2) Expand the scope of use or use of food packaging materials, containers and their additives
(3) New materials and new additives in direct contact with food in tools and equipment for food production and operation
New food raw material registration declaration
1. new food raw material agent declaration service introduction
The new food raw material agent declaration service project (hereinafter referred to as this project) is a series of services provided by Xinanrun to food enterprises in accordance with relevant laws and regulations, such as sample inspection, safety risk assessment, data declaration and so on, aiming to help customers pass the approval of new food raw materials in a good, fast and economical manner.
In this project, the customer provides basic research materials for the declaration of new food raw materials, and Xinanrun is responsible for product declaration, including coordinating sample inspection, safety risk assessment, preparing declaration materials, assisting in organizing expert pre-examination, and submitting materials on behalf of the agent.
2. Project Objectives
Xin'anrun will guide and help food enterprises to obtain approval of new food raw materials declared in accordance with the requirements of laws, regulations and norms.
3. Project Process
New food raw material agent declaration service scheme |
|||
Working phase |
Work Content |
Phased output results |
Time (month) |
0 Pre-assessment |
Before the project starts, the customer truthfully fills in the pre-evaluation questionnaire, and Xinanrun pre-evaluates the project. |
Assess the possibility of starting the project and the intention of the service plan. |
√ |
1 Signing the contract |
The relevant partners of the project agree on matters related to cooperation and reach an agreement. |
Technical Consultation Contract |
√ |
2 Data collection and analysis |
Xinanrun collates and analyzes the original data provided by customers, puts forward additions and amendments, searches for data that are helpful for declaration, the use history of various countries, official food certificates, holds expert demonstration meetings, summarizes and analyzes safety research data, and formulates project plans. |
Project Plan |
√ |
3 Sample inspection |
Contact the inspection unit to carry out variety identification, component analysis, hygienic inspection of three batches of samples, toxicological evaluation, microbial drug resistance and toxin production ability test (microbial), etc., prepare the required data for inspection, submit the inspection data and samples, track the inspection progress, deal with the problems in the inspection, and review the inspection report. |
Related Inspection Report |
/ |
4 Safety assessment |
Contact the safety risk assessment agency to conduct risk assessment of the product, prepare risk assessment data, track the assessment progress, deal with the problems arising from the assessment, and review the assessment report. |
Security Risk Assessment Report |
/ |
5 Preparation and declaration of information |
According to the requirements of relevant laws and regulations, collate research materials, relevant reports, supporting documents and other materials, prepare application materials, and organize experts to conduct pre-examination to ensure that the materials are correct and submitted to the State Health and Family Planning Commission. |
Notice of Acceptance of Application Materials |
√ |
6 Data correction |
After submitting the application materials, track the progress of the application, and receive the review opinions, supplement the materials according to the opinions, reply on the spot as required, finally pass the approval, and the Health Planning Commission issues an announcement. |
Health Planning Commission Announcement |
/ |
Total |
Including data collection and analysis, data preparation and reporting |
4 months |
|
Note: 1. If new regulatory documents involving application materials are issued during the implementation of the project, the application materials need to be adjusted, and the time limit is not covered within the planned time.
2. The execution sequence of phases can be adjusted or parallel according to the actual situation of the project.
4. health food registration filing service
① Registration, filing, change, continuation and technology transfer of domestic health food
Imported health food registration, filing, change, continuation, technology transfer
③ Cleaning and replacement of the Health Food Approval Certificate approved by the former Ministry of Health
④ Preparation of a full set of application materials, pre-review by review experts, acceptance and supplementary information according to review opinions.
⑤ Review and appeal of products not approved
5. infant formula milk powder product formula agent registration service
The infant formula milk powder product formula registration technical support (consultant) service project (hereinafter referred to as the registration project) is a formula registration technical support (consultant) service provided by Xin Anrun according to relevant requirements to solve a series of registration problems such as difficulty in organizing formula registration materials, unclear thinking, lack of expert guidance, complicated online application, etc.
The registration project is led by the customer to organize the application materials. Fumeite provides technical support and audit assistance for the customer's registration work, including technical evaluation of the customer's material preparation, guidance and rectification, assistance in the production of registration documents, organization of expert pre-audit, and agency declaration.
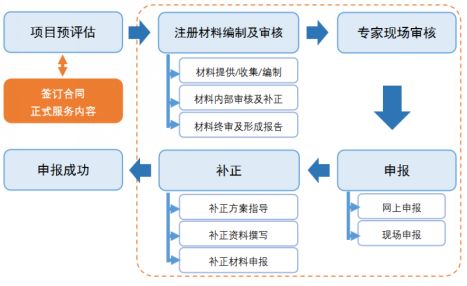
The project team will follow the "Food Safety Law" and its implementation regulations, "Administrative Measures for the Registration of Infant Formula Milk Powder Products" and related supporting documents, "Infant Formula Milk Powder Production License Review Rules (2013 Edition)" and other laws According to the requirements of regulations, rules and regulations, guide and help infant formula milk powder manufacturers with formula registration conditions to successfully obtain the infant formula milk powder product formula registration certificate within a certain time limit.
Xinangrun provides technical support and audit assistance for the registration of customers, which may include the following:
1. Counseling and rectification:
Coaching customers to identify, organize and typeset effective materials.
Coaching customers to solve technical problems in material organization according to the results of internal material audit.
2, sample inspection guidance
Customers can inspect themselves or entrust a third-party inspection agency to carry out the inspection.
Self-inspection, to assist customers to form inspection reports.
Commissioned inspection, the whole process is responsible for the docking of testing institutions, to assist customers to complete the inspection report.
3, the preparation of registration documents
In accordance with the requirements of the "Administrative Measures for the Registration of Infant Formula Milk Powder Products (Trial)" and its supporting documents, assist customers in sorting out and making registration application documents (electronic version).
4, expert audit (optional service)
Organize relevant experts in the industry to conduct a comprehensive review of the customer company's site and related application materials, and issue audit opinions.
5, material declaration
Improve the registration materials and report online and on-site to the relevant departments.
6, tracking feedback
Follow-up and feedback of the registration review process (review progress tips, correction of guidance materials, etc.).
Special note: the consultant service provided is only at the technical level, and does not support the supplement of test data (except documents), process materials and customer specification documents, and does not support the fabrication of materials.
OUR SERVICES
1. food contact materials and food registration
(1) Food contact materials regulatory consultation
(2) Declaration of Conformance Issued
(3) Translation of regulations
(4) Customer product customization consulting report
(5) Experiment commission and test supervision
(6) Communication between Chinese authorities/industry experts
(7) Customer product customization consulting report
(8) Compliance training
(9) Supplier management
2. new food raw materials registration declaration
(1) Food raw material compliance assessment
(2) Identification of new food raw materials
(3) Pre-evaluation of new food raw materials registration
(4) Registration declaration of new food raw materials
(5) Testing supervision of new food raw materials
(6) Health food registration and filing
(7) Registration of infant formula
3. new food raw materials registration declaration
(1) Domestic health food registration, filing, change, continuation, technology transfer
(2) Imported health food registration, filing, change, continuation, technology transfer
(3) Cleaning and replacement of the Certificate of Approval of Health Food approved by the former Ministry of Health
(4) Preparation of a full set of application materials, pre-review by review experts, acceptance and supplementary information according to review opinions
(5) Review and appeal of disapproved products








