US FDA certification
Service Item

A. general food FDA certification
The FDA's regulatory agency for food, agricultural products, and seafood is the Center for Food Safety and Nutrition, whose responsibility is to ensure that the American food supply is safe,Clean, fresh and clearly marked. The main monitoring points include:
1. Food freshness; 2. Food additives; 3. Food biotoxins and other harmful ingredients; 4. Seafood safety analysis; 5. Food labeling;6, food after the market tracking and warning
The FDA of the United States has required foreign companies exporting food (and animal food) to the United States to register with the FDA since December 12, 2003. If the products of these companies are not registered, they cannot land in the United States. The companies referred to hereIncluding product production company, packaging company, wholesale company packaging company and so on.
B. canned food FDA certification
1. A copy of the business license of the applicant or manufacturer; 2. A copy or scanned copy of the product test report (English version);3. English product instructions; 4. List of models and specifications; 5. List of product ingredients; 6. Description of sterilization methods;7. Process flow diagram;8. Product formula proportion table
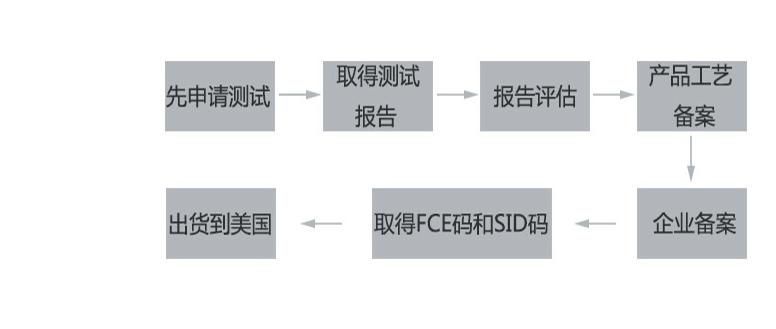
Canned food FDA certification cycle:
From applying for the test to obtaining the registration code, it usually takes about 2 months. It is urgent and can be obtained in about a month.
(NoteCanned food is regarded as junk food in the United States, also known as corrupt food. It is generally concerned by FDA and customs. If canned food exported to the United States is not registered by FDA, it will be detained immediately without any room for accommodation,and may also be fined)
1. English name of the product; 2. Picture; 3. English name of the company; 4. English company address; 5. Telephone; 6. Contact person; 7. E-mail; 8. Website
FDA certification testing standards for food contact materials:
1. Paper Products Standard U.S.FDA CFR 21 176.170
2. Requirements for Organic Coatings, Metals and Electroplated Products U.S.FDA CFR 21 175.300
3, food container sealing ring, sealing progenitor gasket requirements U.S.FDA CFR 21 177.1210.
4. Ceramic, glass, enamel utensils U.S.FDA CPG 7117.06.07
5. Metal Requirements U.S.FDA CFR 175.300 & CPG 7117.05
Cosmetics FDA certification information:
1. Application form; 2. Product labels and instructions; 3. Safety testing and test report, and effectiveness (functionality) test report; 4. Brief introduction of product formula and equipment technology; 5. Toxic skin irritation test report; 6. Provide appropriate samples consistent with the application documents; 7. Product name and composition list
Cosmetics FDA certification test items:
1. Heavy metal test; 2. Microbiological test; 3. Skin irritation test; 4. Physical and chemical composition analysis; 5. TRA toxicological evaluation; 6. Component label review; 7. Antiseptic efficacy test
FDA Registration Requirements for Cosmetics:
1. Review of cosmetic ingredients and their content;
Modifying cosmetic labels to comply with FDA labeling reviews;
3. Registration of cosmetics production enterprises;
4. Registration of cosmetic ingredients; |

|
E. FDA OTC certification
For OTC products under the US FDA, the following services can be provided: • apply for a Dun & Bradstreet number; • Site registration; • Apply for NDC; • FDA product filings; • Product label review; |
F. Medical Device Products under FDA
The following services are available: • The manufacturer is registered with FDA; • FDA product registration; Product listing registration (510 form registration); • Product go-to-market review approval (PMA review)
|
• Product go-to-market review approval (PMA review)
Chemicals exported to the United States are subject to the requirements of the US Toxic Substances Control Act (TSCA). The U.S. Occupational Safety and Health Administration (OSHA) is responsible for the classification, labeling, and chemical safety data sheets of chemical substances in the workplace, and hazardous chemicals must comply with the requirements of the Hazard Transmission Standard (HCS) issued by OSHA. In addition, in the United States, hand sanitizer is an over-the-counter (OTC) for local disinfection. Like anti-dandruff shampoo, fluoride toothpaste, sunscreen and other products, it is regulated by the Food and Drug Administration (FDA). Exporting hand sanitizer to the United States also requires FDA certification. FDA certification will require an NDC number. Domestic manufacturers must apply for the corresponding NDC number for each package OTC before exporting to the United States. NDC(National Drug Code National Drug Verification Number) is the identification symbol of drugs as common commodities. FDA of the United States regularly edits the NDC system index, and enters the NDC number and registration information as the program to enter the Drug Registration and Listing System (DRLS) database. It includes all prescription drugs and some screened over-the-counter drugs and islet drugs. According to Section 510 of the United States Code of Federal Regulations, the NDC of each drug listed has 10 numbers and consists of three parts, namely, manufacturer number, product number and package model. The first part is the manufacturer number provided by FDA, which refers to the manufacturer that produces or sells drugs. The second part is the product number; mark the characteristics, dosage form and configuration of the product. The third part is the packaging code. The labels of the second and third parts are provided by the manufacturer. The structure of the NDC number consists of one of the following forms, I .e., 4-4-2, 5-3-2, or 5-4-1, e.g., 62684-010-10, I .e., 5-3-2.
Products with NDC can be listed in the form of drugs in the United States. Vendors can learn about the efficacy and characteristics of the products according to this number in order to order the drugs. At the same time, FDA also manages the products according to this number. The disadvantage of NDC is that there is no reference table for drug trade names and common names. Products with NDC can be listed in the form of drugs in the United States. Vendors can learn about the efficacy and characteristics of the products according to this number in order to order the drugs. At the same time, FDA also manages the products according to this number.
(II) US EPA Registration Services
What is a U.S. Agent/U.S. Agent?
Any foreign agency engaged in the manufacture, preparation, reproduction, compounding, or processing of equipment or products imported into the United States must identify a U.S. agent (U.S. agent) for the agency.
Information about the U.S. agent of a foreign agency is submitted electronically using the FDA Unified Registration and Listing System (FURLS system) and is part of the agency registration process. Each foreign agency may designate only one U.S. agent. A foreign agency may also, but need not, designate its U.S. agent as its official agent. The foreign agency should provide the name, address, telephone and fax numbers, and e-mail address of the U.S. agent.
The identified U.S. agent will need to complete an automated process to confirm that they have agreed to act as a U.S. agent. The automated process forwards the email verification request to the U.S. agency. They will be asked to confirm her/his consent to act as a representative/liaison on behalf of the foreign institution. If the U.S. agent refuses to consent (or does not respond within 10 business days), the official correspondent/owner operator of the foreign agency will be notified and a new U.S. agent must be appointed to meet regulatory obligations.
Responsibilities of U.S. Agents
S. agent must reside in the United States or have a place of business in the United States. U.S. agents cannot use a post office box as an address. The US agent cannot use only the answering service. They must be available to answer the phone or have employees answer the phone during normal working hours.
The liability of the US agent is limited and includes:
1. Assist FDA to communicate with foreign agencies,
2. Answer questions about equipment imported into the United States or foreign institutions that intend to import into the United States,
3. Assist FDA in arranging inspections of foreign institutions
If FDA is unable to contact the foreign agency directly or promptly, FDA may provide information or documents to the U.S. agency, and such action should be equivalent to providing the same information or documents to the foreign agency.
Please note that agents in the United States have no liability associated with adverse event reporting under the Medical Device Reporting Regulations (21 CFR Part 803), or the filing of 510(k) premarket notifications (21 CFR Part 807, Part E).









