National Service Hotline:
-
-
-
-
-
Contact

One-stop chemical compliance services, providing chemical compliance consulting services
Master Document Registration
The main document of medical device is a kind of technical document, which mainly involves the raw materials, components, design, manufacturing process, performance testing, clinical research data and other key technical information of medical device. The main document registration of medical devices is to encourage medical device innovation, simplify the registration process, and protect the intellectual property rights of the main document owner.
1. Applicable Regulations for Registration of Main Documents of Medical Devices
Announcement of the State Food and Drug Administration on Registration of Main Documents of Medical Devices
Measures for the Administration of Medical Device Registration
Administrative Measures for the Registration of In Vitro Diagnostic Reagents
Medical Device Registration Application Data Requirements and Approval Document Format
《In vitro diagnostic reagent registration declaration data requirements and approval document format
Technical Guide for Electronic Submission of Applications for Medical Device Registration
Application of medical device registration review guidelines
2. Application scope of registration items of main document of medical device
The content of the main document of medical devices mainly involves the raw materials, components, design, manufacturing process, performance testing, clinical research data, etc.
For the main documents cited by medical device registration applicants in the registration, change, and clinical trial approval of imported second-class, third-class and domestic third-class medical devices (including in vitro diagnostic reagents), the drug administration of each province, autonomous region, and municipality directly under the Central Government may According to the actual situation, refer to the announcement to carry out the registration of the main document of the second-class medical device in the country.
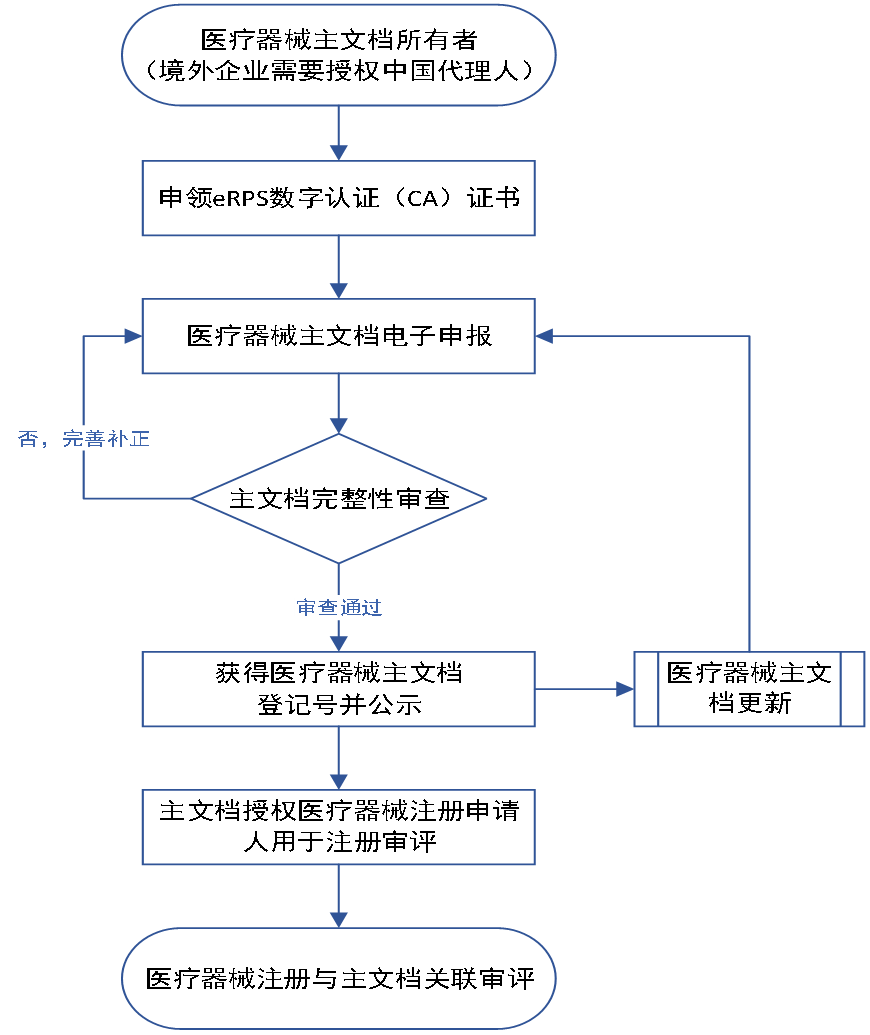
3. Medical Device Master Document Registration Process
The Medical Device Technical Review Center of the State Drug Administration shall establish a registration platform and database for the main document of medical devices, and the owner of the main document shall submit the registration information of the main document as required, and obtain the registration number of the main document after registration. The instrument review center will review the main document data together after the relevant application for registration of the associated medical device is submitted.
4. Medical device main document registration data list
Regional management information: application forms, supporting documents, compliance/authenticity statements, etc;
General information: a comprehensive description of the device and operating principle;
Non-clinical research information: select applicable research items according to registration items, such as physical and mechanical properties, chemical/material characterization, biocompatibility and toxicology evaluation, cleaning and disinfection confirmation, etc.
Clinical evaluation data: if applicable, refer to the relevant data of clinical evaluation guidelines;
Quality management system procedures: manufacturing information and production site information.
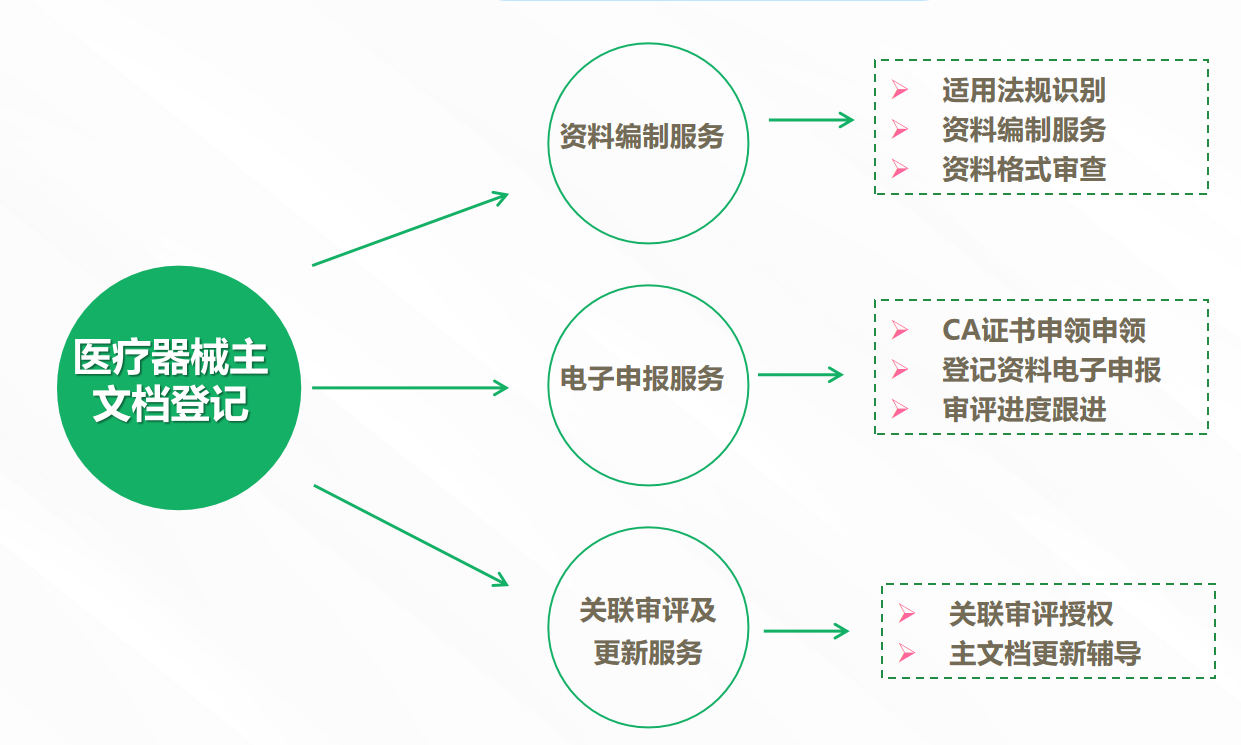
5. Service Content
National Service Hotline:
Beijing Headquarters: D10, Floor 4, Block A, Borui Building, No.26, Dongsanhuan North Road, Chaoyang District, Beijing, China
Tel: 86-010-6471 0683
Shanghai: Room D308, Zhongyi International Building, Puxing Highway 800, Minhang District, Shanghai
Tel: 86-021-3478 3993
Guangzhou: Room 201, Xinying Technology Park, No. 313 Jinxin Avenue, Panyu District, Guangzhou
Tel:400 660 1329
E-mail:nar@china-reach.net
Copyright: New Anrun (Beijing) Consulting Co., Ltd.







